Storing controlled substances properly isn’t just about following rules-it’s about keeping patients safe and protecting your team from serious consequences. Every year, thousands of pills, patches, and injections go missing from hospitals, clinics, and pharmacies. Some end up in the wrong hands. Others are stolen by staff. The result? Patients get less medicine when they need it. Staff face investigations, loss of licenses, or even jail time. And facilities pay hundreds of thousands in fines.
Why This Matters More Than Ever
In 2025, the DEA requires real-time inventory tracking for any facility handling more than 10kg of Schedule II drugs annually. That’s not a suggestion-it’s the law. And inspections are up 37% since 2019. If your storage system doesn’t log every touchpoint, you’re already behind.
Diversion doesn’t always look like someone stealing a bottle. It’s often subtle: a nurse swapping a fentanyl vial for saline, a pharmacist forgetting to log a return, a technician carrying a purse into the pharmacy. These aren’t just mistakes-they’re gaps in your system. And they’re how most large-scale incidents start.
What Counts as a Controlled Substance?
Not all pain meds are the same under the law. The DEA classifies them into five schedules based on abuse potential and medical use:
- Schedule II: Oxycodone, fentanyl, hydromorphone, methadone, Adderall. High abuse risk, severe dependence. Must be stored in a locked, bolted cabinet with dual control.
- Schedule III: Hydrocodone with acetaminophen, ketamine, buprenorphine. Moderate abuse risk. Still requires locked storage-even if state law says otherwise.
- Schedule IV: Benzodiazepines like lorazepam, diazepam, tramadol. Lower abuse risk, but still tracked.
- Schedule V: Cough syrups with codeine, limited amounts of opiates. Often overlooked, but still regulated.
Any drug in these categories must be accounted for from the moment it arrives until it’s given to a patient-or destroyed. Missing even one tablet can trigger a DEA investigation.
Physical Storage: The First Line of Defense
Locks aren’t enough. You need layers.
For large facilities: Automated Dispensing Cabinets (ADCs) are now the standard. These are smart cabinets-like ATMs for meds-that require two forms of authentication: a badge and a biometric scan (fingerprint or retina). Every time someone takes a drug, it’s logged with their name, time, and dosage. These systems cut diversion incidents by 73% when used correctly.
For smaller clinics or rural hospitals: If you can’t afford an ADC, use a double-locked cabinet. One lock is a key lock. The other is a combination lock. Both keys or codes must be held by different people. No one person can open it alone. This is called dual control. It’s old-school, but it works.
Storage areas must be:
- Locked at all times-even overnight
- Not visible from public areas
- Restricted to pharmacy and authorized clinical staff only
- Monitored by surveillance cameras (if possible)
And here’s something many miss: no personal bags, purses, or coats allowed in medication storage areas. In 31% of diversion cases, people hid drugs in their belongings. Make this a strict policy. Enforce it. Every day.
Process Over Hardware: The Hidden Risks
Even the best cabinet won’t help if your processes are sloppy.
The biggest risk points aren’t the vaults-they’re the handoffs:
- When a pharmacist refills an ADC manually
- When a nurse takes floor stock from a central cabinet
- When a drug is returned after a patient doesn’t need it
- When waste is disposed of
Manual processes are the weak spot. If you’re writing down transactions on paper, you’re inviting error-and exploitation. Every time you bypass an electronic log, you create a blind spot.
Here’s what works:
- Always use electronic logs for transfers between vault → ADC → patient
- If you must do a manual refill, require two staff members to witness and sign off
- Never allow a single person to handle both ordering and dispensing
- Review daily logs for outliers: same person taking high-dose opioids every shift? That’s a red flag
At Mayo Clinic, they found that facilities with daily pharmacist reviews of dispensing logs reduced diversion by 89%. It’s not complicated. Just consistent.
Training and Culture: The Real Game-Changer
Technology helps. But people stop diversion.
Most staff don’t set out to steal. But they might look the other way. They might think, “It’s just one vial.” Or they might resent new rules and work around them.
Effective programs don’t just train-they build culture.
- Hold mandatory training every six months-not just when new software rolls out
- Include all staff, not just pharmacists. Nurses, anesthesiologists, and even cleaners need to know what’s expected
- Use real examples: “Last year, a nurse diverted 200 fentanyl doses. She was caught because a colleague noticed she was always the one returning empty vials.”
- Make reporting easy. Anonymous hotlines work. So do peer-to-peer check-ins
One hospital in Glasgow reduced incidents by 74% after banning bags and adding dual authentication. But it took three training sessions and two months of firm enforcement before staff stopped complaining.
What to Do When You Find a Problem
If you spot something odd-a missing vial, a pattern of late-night refills, a staff member who always “accidentally” wastes the same drug-don’t wait.
Follow this:
- Document everything: dates, times, names, logs, camera footage
- Report to your pharmacy director or compliance officer immediately
- Do not confront the person yourself
- Initiate an internal review per your facility’s policy
- If confirmed, report to the DEA within 24 hours
Delaying a report is a violation. The DEA doesn’t care if you thought it was “just a mistake.” If you knew or should have known, you’re liable.
Costs and Alternatives: What Fits Your Facility?
ADCs cost $45,000-$75,000 per unit. Annual maintenance adds 15%. That’s out of reach for many small clinics.
But you don’t need an ADC to be compliant.
Here’s a cost-effective plan for smaller settings:
- Use a double-locked cabinet (key + combination)
- Assign two staff members to hold keys/codes
- Log every access in a secure digital spreadsheet (Google Sheets won’t cut it-use a HIPAA-compliant app)
- Require two signatures for any manual refill or return
- Conduct weekly surprise audits
Yes, this takes more time. A 2022 Mayo Clinic study found manual systems require 37% more staff hours. But it’s cheaper than a lawsuit.
And remember: the cost of a single diversion case? Up to $287,000 in patient testing, legal fees, and lost licenses.
What’s Coming Next
AI is starting to change the game. Hospitals like Johns Hopkins are using machine learning to spot patterns-like a nurse who refills opioids at 3 a.m. every Tuesday. These systems flag anomalies in real time, with 92% accuracy and 63% fewer false alarms.
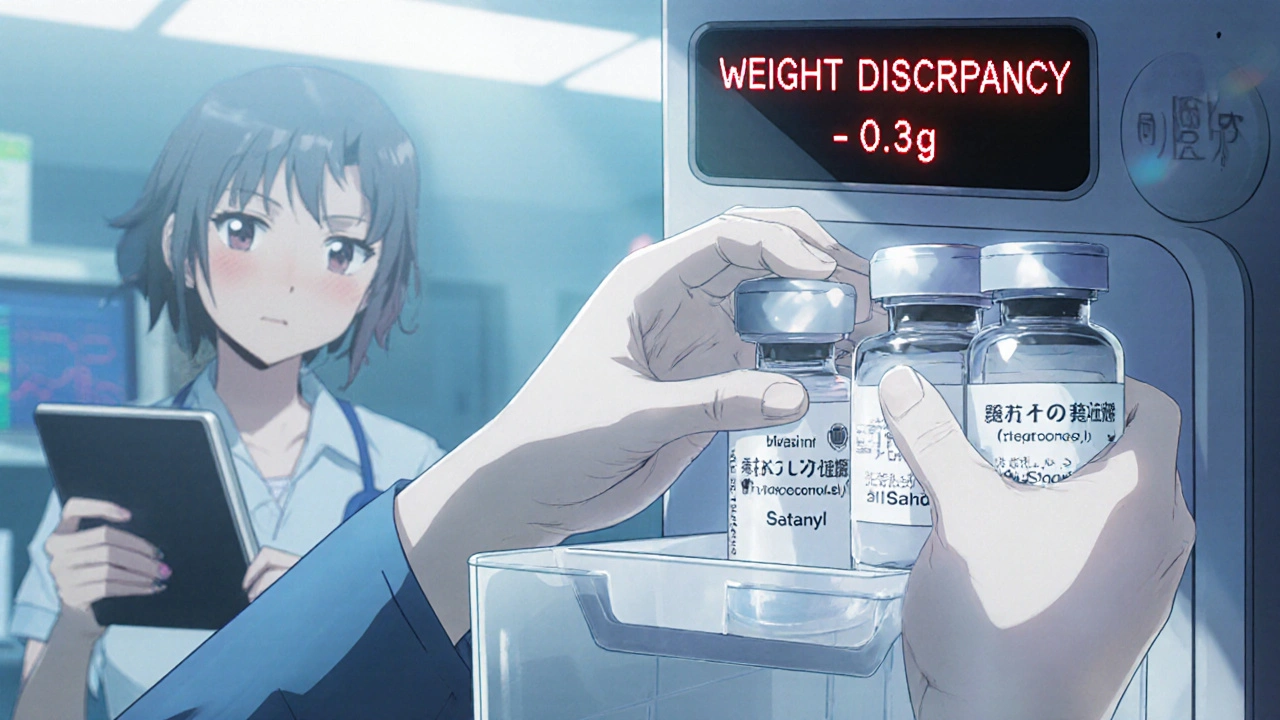
ASHP is also updating its guidelines in mid-2024 to address a new trend: using saline vials to replace stolen opioids. Staff flush the real drug, then document it as “waste.” The saline stays in the bin. It’s hard to catch-unless you’re checking vial weights or using smart bins that scan contents.
The message is clear: if you’re still using paper logs, you’re running a high-risk operation.
Final Checklist: Are You Protected?
Use this to audit your storage setup:
- ☐ All controlled substances are stored in locked, access-controlled areas
- ☐ No personal bags or items allowed in storage zones
- ☐ Dual control is enforced for all access (two people, two keys)
- ☐ Every transaction is logged electronically
- ☐ Daily logs are reviewed by a pharmacist for outliers
- ☐ Staff are trained every six months with real-case examples
- ☐ An anonymous reporting system is active and promoted
- ☐ Waste disposal is witnessed and documented
- ☐ Cameras cover storage areas (if possible)
- ☐ You’ve reviewed DEA’s 2023 real-time tracking requirement
If you checked all 10 boxes-you’re ahead of 60% of facilities.
What Happens If You Don’t?
DEA fines average $187,500 for storage violations. Two in five cases involve inadequate security. But the real cost? Loss of trust. Patients stop believing their meds are safe. Staff stop believing their workplace is ethical. And regulators start watching you closely.
This isn’t about compliance. It’s about integrity.
Storing controlled substances right doesn’t make you a cop. It makes you a guardian.
Can I store Schedule III drugs in the same cabinet as Schedule IV drugs?
Yes, but only if the cabinet meets the highest security standard required for any drug in the group. Schedule III drugs require the same level of control as Schedule II-dual access, locked storage, and full documentation. Mixing them doesn’t lower the rules. Always follow the strictest requirement.
Do I need to report a missing pill if it’s just one?
Yes. Even one missing tablet must be reported to your compliance officer and, if confirmed as theft or loss, to the DEA within one business day. The DEA doesn’t judge quantity-they judge accountability. A single unexplained loss can trigger a full audit.
Can nurses keep controlled substances in their personal lockers?
Absolutely not. Personal lockers are never permitted for storing controlled substances. This is a direct violation of DEA regulations and is a common method used in diversion cases. All meds must remain in designated, secured storage areas under institutional control.
Is it okay to use a standard office safe for controlled drugs?
No. Office safes are not DEA-compliant. They lack audit trails, dual-access controls, and are often not bolted down. Only cabinets designed for pharmaceutical use-with electronic logging and physical security features-meet regulatory standards.
How often should we audit our controlled substance inventory?
Daily electronic logs should be reviewed by a pharmacist for anomalies. A full physical inventory must be done at least monthly. Some high-risk areas, like operating rooms, require weekly counts. Surprise audits are also recommended quarterly to deter internal theft.
What if my facility can’t afford an automated dispensing cabinet?
You can still be compliant with manual systems-but you must compensate with stricter procedures. Use dual-control locked cabinets, require two signatures for every transaction, log everything digitally (not on paper), and conduct daily reviews. It’s more labor-intensive, but it’s legal and effective if done rigorously.
Can I dispose of unused controlled substances in the regular trash?
Never. Controlled substances must be destroyed under witness, using an approved method like a DEA-registered reverse distributor or an on-site destruction unit. Documentation of the destruction, including witness names and dates, must be kept for at least two years.

 Medications
Medications
Kartik Singhal
November 20, 2025 AT 16:18Oh wow, another corporate compliance pamphlet masquerading as ‘practical guidance’ 🤡
Let me guess-you also think biometric scans will stop a nurse who’s already addicted? 🤨
Meanwhile, the real problem? Pharma companies flooding the market with opioids, then screaming ‘diversion!’ when the system collapses.
And don’t get me started on ‘dual control’-it’s just theater. Someone’s always got a spare key or a backdoor.
Also, why is no one talking about how these cabinets cost more than a used car? 🤑
Small clinics? They’re just gonna keep using paper logs until someone dies. Again.
And the DEA? Please. They’re busy chasing college kids with vape pens while real theft happens in plain sight.
TL;DR: This is performative security. We’re all just actors in a broken system.
Also, why is there a whole section on ‘what happens if you don’t?’ Like we don’t already know? 😴
Chris Vere
November 21, 2025 AT 02:25It is indeed a matter of great gravity that we consider the stewardship of controlled substances with the utmost seriousness
Every vial represents not merely a chemical compound but a human life in potential
The institutional structures described herein reflect a moral imperative beyond mere regulation
When we fail to secure these substances we do not merely violate policy we violate the covenant between healer and patient
It is not enough to comply with the law we must embody its spirit
May we all strive to be guardians not merely of inventory but of dignity
Thank you for this thoughtful exposition
It is rare to encounter such clarity in a field so often clouded by bureaucracy
Pravin Manani
November 22, 2025 AT 07:21Let’s unpack the operational risk architecture here - the real leverage point isn’t the ADCs or dual control per se, it’s the cognitive load on frontline staff.
When you layer compliance protocols onto already overburdened workflows, you don’t reduce diversion - you create compliance fatigue.
And that’s where the real vulnerability emerges: not in the lock, but in the mental model of the nurse who’s been on 12-hour shifts for 5 days straight.
You can mandate biometrics, but if the system doesn’t account for human degradation, you’re just automating the illusion of safety.
Also - the ‘no personal bags’ rule? Valid. But it’s a band-aid on a systemic wound.
The root cause? Understaffing, burnout, and lack of psychological safety to report concerns.
That’s why 74% reduction in Glasgow worked - it wasn’t the locks, it was the culture shift.
And yes, AI anomaly detection is promising, but only if it’s used for coaching, not punishment.
Otherwise you’re just building a panopticon with better UX.
Bottom line: Tech enables, but culture sustains.
Fix the environment, not just the cabinet.
Mark Kahn
November 23, 2025 AT 13:27This is actually really helpful - thank you for laying it all out so clearly!
I work in a small rural clinic and we’re just starting to get our act together with controlled meds.
Our old cabinet was a joke - one lock, no logs, and someone’s lunchbox was always in there 🤦♂️
We just got a double-lock cabinet this week and assigned two staff to hold the keys - no more single-person access!
Also started doing surprise audits every Friday - turns out one of our techs was taking a few oxycodone tabs for his back pain.
He didn’t even realize it was a big deal until we talked.
We’re getting him help now - not firing him.
That’s the thing - it’s not about being the police. It’s about being the team that catches people before they fall.
You guys are doing great work. Keep it up!
Daisy L
November 25, 2025 AT 05:10AMERICA IS BEING STOLEN FROM US!!!
THEY’RE STEALING OUR OPIOIDS AND GIVING THEM TO ILLEGAL IMMIGRANTS AND DRUG ADDICTS!!!
WHY IS NO ONE TALKING ABOUT THIS?!?!
THE DEA IS ASLEEP AT THE WHEEL!!!
AND DON’T EVEN GET ME STARTED ON THE PHARMA COMPANIES!!!
THEY’RE ALL IN ON IT!!!
THEY MAKE THE DRUGS, THEN THEY MAKE THE RULES, THEN THEY PROFIT FROM THE FINES!!!
WE NEED A WALL AROUND THE PHARMACY!!!
AND BAN ALL BAGS!!! EVERY SINGLE BAG!!!
THEY’RE HIDING DRUGS IN PURSES, IN SHOES, IN DIAPERS!!!
WE NEED ARMED GUARDS!!!
AND A NATIONAL DATABASE!!!
AND A TRUMP-STYLE AUDIT!!!
AND NO MORE ‘EMPATHY’!!!
JUST LOCK THEM UP!!!
AMERICA FIRST!!!
MAKE OUR MEDS SAFE AGAIN!!!
🇺🇸💊🔫
Anne Nylander
November 26, 2025 AT 20:59OMG this is sooo helpful!!
I just started as a nurse and I had no idea how serious this stuff is!
My hospital uses ADCs but I never thought about the logs or who’s checking them!
Now I’m gonna start paying attention and if I see somethin weird I’m gonna say somethin!
Also I just told my coworker to stop leaving her purse near the med cart-she was like ‘why?’ and I was like ‘BECAUSE DRUGS!’ 😅
Thanks for remindin me we’re all in this together!!
PS: I typed this on my phone so sorry if I misspelled stuff!!
Noah Fitzsimmons
November 28, 2025 AT 11:58Oh wow. A 10-page essay on how to lock a cabinet.
Let me guess - you also think putting a sticker on the door that says ‘No Bags’ will stop a nurse who’s been stealing fentanyl for three years?
You know what’s really funny? You mention ‘31% of diversion cases involve purses’ like that’s some shocking revelation.
It’s not. It’s the most obvious thing in the world.
And yet you spent 800 words telling people to stop bringing their bags.
Meanwhile, the real culprits? The hospital administrators who cut staffing by 40% and then wonder why people start stealing.
But sure. Let’s blame the nurse’s purse.
Classic. Just… classic.
Clifford Temple
November 29, 2025 AT 18:40THIS IS WHY AMERICA IS FALLING APART.
WE’RE LETTING PEOPLE STEAL MEDS AND THEN WE WRITE 5000 WORDS ABOUT LOCKS AND LOGS?
WHY NOT JUST LOCK THE NURSES IN THE PHARMACY?
WHY NOT HAVE A BACKGROUND CHECK FOR EVERY SINGLE EMPLOYEE?
WHY NOT ARM THE PHARMACISTS?
WHY ARE WE STILL USING ‘DUAL CONTROL’ IN 2025?
WE NEED A DIGITAL ID CARD THAT TRACKS EVERY BREATH THEY TAKE.
AND IF THEY TOUCH A VIAL WITHOUT PERMISSION - ZAP THEM.
THIS ISN’T A TRAINING MANUAL.
THIS IS A WAR.
AND WE’RE LOSING.
AND YOU’RE WRITING ABOUT ‘CULTURE’?
WE NEED MILITARY-LEVEL SECURITY.
NO MORE ‘EMPATHY’.
NO MORE ‘HELP THEM’.
JUST LOCK. IT. UP.
Corra Hathaway
November 30, 2025 AT 17:22Y’all are overthinking this 😘
Just treat people like humans. Be kind. Say thank you. Check in.
Most people who steal drugs aren’t evil - they’re hurting.
Maybe they’re in pain. Maybe they’re scared. Maybe they’re just tired.
Instead of policing every movement, build a place where people feel seen.
That’s how you stop theft - not with cameras, not with biometrics, but with connection.
And yes, you still need locks. But locks without love? Just a fancy cage.
Also - I love that Mayo Clinic did daily reviews. That’s the kind of quiet leadership that changes lives 💖
Keep being the good ones. We need you.
Shawn Sakura
December 2, 2025 AT 10:25Thank you for this comprehensive and meticulously structured guide. It is both a practical resource and a moral compass in an increasingly complex healthcare landscape.
While I appreciate the emphasis on technological solutions such as ADCs, I would like to respectfully underscore the critical importance of human factors - particularly the psychological and emotional toll on frontline providers.
It is not merely a matter of procedural compliance; it is a matter of preserving the sanctity of the healing relationship.
Even the most advanced biometric system cannot compensate for a culture of silence.
Therefore, I propose that we institutionalize monthly peer-review circles, where staff may anonymously reflect on ethical dilemmas, without fear of reprisal.
Additionally, I would recommend integrating mindfulness practices into shift handoffs - a mere 90 seconds of silent breathing before accessing controlled substances may reduce impulsive behavior.
Let us not mistake vigilance for virtue.
Let us cultivate integrity - not just through policy, but through presence.
Thank you again for your leadership in this vital domain.